My novel-in-progress, Red Soil Through Our Fingers, takes place in a farming settlement that is part of a Mars colony. As I wrote Draft Zero, there was a lot of handwavium going on with respect to various technical details — the focus on just finishing the story. Now that I’ve progressed about a quarter of the way into Draft Alpha, I’m needing to clean up some inconsistencies and gaps. Today I figured out a piece of the story world that is good for me to know as I construct the novel, but probably won’t make it explicitly into the next of the work: how various molecules necessary for both human life and agriculture are going to be circulated on this Mars colony.
After some poking around the interwebs and a little thought, it seems to me that four gases are needed as inputs into my farm system, with their colors as labeled on the diagram below: nitrogen (green), oxygen (light blue), carbon dioxide (yellow), and hydrogen (red).
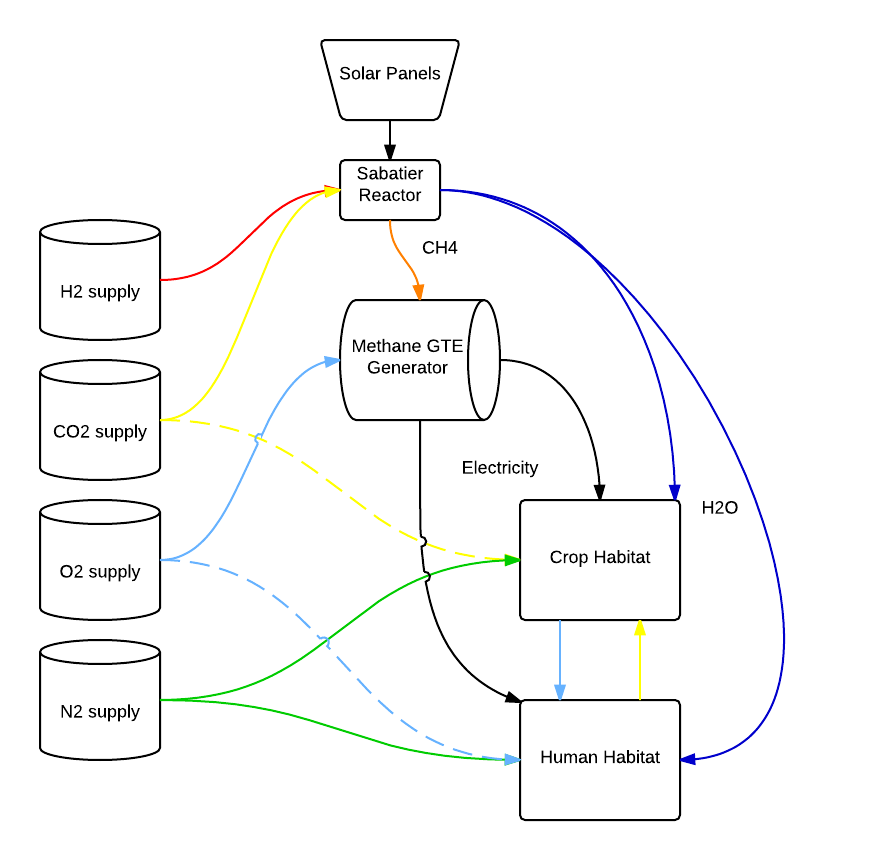 The CO2 I assumed could be compressed from the Martian atmosphere, and the oxygen extracted from crushing and refining the regolith, which is rich in oxdides and other oxygen-bonded molecules. The nitrogen and hydrogen, as far as I am aware, would have to be imported, likely making them quite expensive for our hypothetical farmer.
The CO2 I assumed could be compressed from the Martian atmosphere, and the oxygen extracted from crushing and refining the regolith, which is rich in oxdides and other oxygen-bonded molecules. The nitrogen and hydrogen, as far as I am aware, would have to be imported, likely making them quite expensive for our hypothetical farmer.
Oxygen and CO2 can be exchanged between crop plants and humans, with some occasional replenishment due to losses or expansion of either the plant or human population of the farm.
Similarly, water can be recycled with very minimal closed-circuit losses (as the ISS has already demonstrated). However, losses in a large farm with many biological and mechanical components are likely. The additional problem is that water is very heavy, and importing it from Earth or water-rich asteroids is likely to be very expensive. The Sabatier reaction, also used on the ISS, manufactures methane and water from CO2 and hydrogen gas: CO2 + 4 H2 → CH4 + 2 H2O + energy. Though the reaction is exothermic, a jolt of activation energy is required.
The Sabatier reactor and all-low power electronics, emergency power, etc could be provided by solar panels. However, I’m skeptical that solar alone could power a large farm operation, given the weaker sunlight intensity on Mars. At least, not without using large amounts of land area, or orbital stations, etc. Possible, but remember, I’m not talking about a large government operation with a huge base and unlimited resources (the settlers in Kim Stanley Robinson’s Red Mars afterall, had their own military-grade nuclear reactor). This is what a sole proprietor level farm might reasonably be able to afford with access to some sort of start-up capital loan.
I decided that a small, methane-powered, gas turbine engine would make an easily scalable power solution that would also make use of the methane produced from the Sabatier reaction (a molecule that the ISS actually considers a waste product, and dumps). The resulting combustion would result in more CO2 and H2O, plus some waste gases, but I didn’t label those on the diagram. Presumably they could be recovered and used as well.
The company or utility provides the four essential gases, and the farm synthesizes most of what it needs from there.
It’s a first cut conceptual design, which is probably all the detail I need for now.